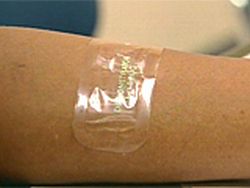
Duragesic Patch – Fentanyl
On July 15, 2005, the Food and Drug Administration (“FDA”) issued a public health advisory to alert patients and their physicians of reported deaths and other serious side effects from overdoses of fentanyl administered through the use of a transdermal patch. These side effects have occured using both the brand name Duragesic Patch, as well as the generic counterparts. The Oshman Firm has been investigating fentanyl litigation for over a year, and while the dangers of this pain killer have been known for at least that long, it is only now that the FDA has taken action.
Fentanyl is a very strong narcotic painkiller. As a result, administering this drug can be very dangerous. Even assuming the transdermal patch works perfectly, the directions for using this drug must be followed exactly as described to avoid death and serious injury from overdoses. In many instances, however, there have been reports from patients and physicians of defective patches which leak fentanyl onto the skin faster than the drug is intended to be released. The result in such cases is an overdose which can cause death and other serious side effects.
If you or someone in your family have experienced the effects of a fentanyl overdose, join our other clients who have retained us in this litigation and be assured of having experienced legal representation. You can contact partner Ted Oshman at 1-800-400-8182 or fill out the questionairre that follows. For your convenience, we have also provided you with the text of the FDA alert at the bottom of this page.
FDA Public Health Advisory
Safety Warnings Regarding Use of Fentanyl Transdermal (Skin) Patches
FDA is investigating reports of death and other serious side effects from overdoses of fentanyl in patients using fentanyl transdermal (skin) patches for pain control. Deaths and overdoses have occurred in patients using both the brand name product Duragesic and the generic product. The directions for using the fentanyl skin patch must be followed exactly to prevent death or other serious side effects from overdosing with fentanyl. These directions are provided in the product label and patient package.
- Fentanyl skin patches are very strong narcotic (opioid) painkillers that may cause death from overdose. The fentanyl skin patch should always be prescribed at the lowest dose needed for pain relief.
- Fentanyl skin patches should not be used to treat short-term pain, pain that is not constant, or for pain after an operation. Fentanyl skin patches should only be used by patients who are already taking other narcotic painkillers (opioid tolerant), and who have chronic pain that is not well controlled with shorter-acting painkillers.
- Patients who are using the fentanyl skin patch and their caregivers should be told about the directions for safe use of the patch and should follow the directions exactly. These directions are provided in the patient package.
- Patients who are using the fentanyl skin patch and their caregivers should be told about safe methods for storage and disposal of used, unneeded or defective fentanyl skin patches. Fentanyl skin patches should be stored in a safe place and kept out of the reach of children. Safely dispose of used, unneeded or defective fentanyl skin patches by folding the sticky side of the patch together (until it sticks to itself) and flushing it down the toilet.
- Health care professionals who prescribe the fentanyl skin patch and patients who use the fentanyl skin patch and their caregivers should be aware of the signs of fentanyl overdose. Signs of fentanyl overdose include trouble breathing or shallow breathing; tiredness, extreme sleepiness or sedation; inability to think, talk or walk normally; and feeling faint, dizzy or confused. If these signs occur, patients or their caregivers should get medical attention right away.
- A patient using the fentanyl skin patch may have a sudden and possible dangerous rise in their body level of fentanyl or have a stronger effect from fentanyl if they: use other medicines that affect brain function; drink alcohol (beer, wine or distilled spirits); have an increase in body temperature or are exposed to heat; or use other medicines that affect how fentanyl is broken down in the body. These factors are described further in the product label.
Some patients and health care providers may not be fully aware of the dangers of this very strong narcotic painkiller. Therefore, FDA is issuing this public health advisory to alert patients and their caregivers and health care professionals by highlighting the following important safety information:
In June 2005 the Duragesic product label was updated to add new safety information in several areas of labeling, and a “Dear Healthcare Professional” letter about these changes was issued by the manufacturer and is available at this link (http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/default.htm). FDA continues to work with the manufacturers of these products to identify and manage factors that contribute to fentanyl overdose from use of the fentanyl skin patch. Updates will be provided as new information is available.
