 Elidel is a medicated cream commonly prescribed to patients for the condition, atopic dermatitis. The medication, approved by the Food and Drug Administration in December of 2001, is now eliciting serious concern regarding adverse and potentially fatal side effects. On January 19, 2006, the FDA approved an updated Black Box warning for Elidel, indicating that the medication may be directly associated with the onset of lymphoma and skin cancer.
Elidel is a medicated cream commonly prescribed to patients for the condition, atopic dermatitis. The medication, approved by the Food and Drug Administration in December of 2001, is now eliciting serious concern regarding adverse and potentially fatal side effects. On January 19, 2006, the FDA approved an updated Black Box warning for Elidel, indicating that the medication may be directly associated with the onset of lymphoma and skin cancer.
What is Elidel?
Elidel is distributed by Novartis Pharmaceuticals Corp. and intended to treat mild to moderate atopic dermatitis in adults and children over the age of two. The medication is advised only to be administered as a last resort, for patients who have been unresponsive or intolerable to all other topically medicated creams and treatment options. Elidel acts as an immunomodulating agent to remedy symptoms of redness, itchiness, and cracking associated with atopic dermatitis.
The medication is advised only to be administered as a last resort, for patients who have been unresponsive or intolerable to all other topically medicated creams and treatment options. Elidel acts as an immunomodulating agent to remedy symptoms of redness, itchiness, and cracking associated with atopic dermatitis.
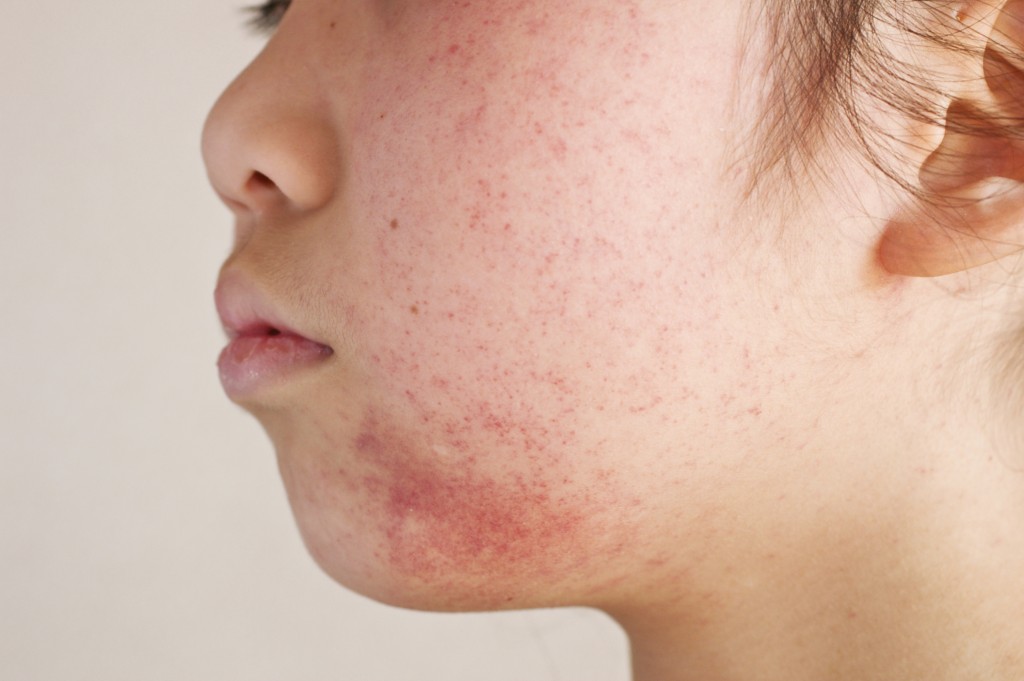
What is Atopic Dermatitis?
Atopic dermatitis is a chronic skin condition characterized by symptoms of itchy and inflamed skin that may come and go in intervals of outbreak and remission. Atopic dermatitis is considered to be one of the most common forms of the skin condition eczema. A person suffering from this type of eczema may experience redness, cracking, inflammation, crusting and scaling of the skin.
Currently, atopic dermatitis affects an alarming 10 to 15 percent of youth in the United States, making children a targeted consumer for this potentially fatal drug.
Though a definite cause of atopic dermatitis has not been established, the condition is suspected to have an immune mediated or allergic factor.
Serious Elidel Injury and Side Effects
The FDA released initial warnings regarding the drug to healthcare providers in March of 2005, when a public health advisory was issued concerning the cancer risks associated with Elidel. The advisory was mandated in response to a request from the FDA’s Pediatric Counsel to include a boxed warning on the medication’s labeling.
This warning has recently elevated to include an updated Black Box warning on Elidel’s labeling, indicating that Elidel patients may have an increased risk for lymph node cancer and skin cancer. The Black Box warning is considered to be the most severe cautionary issued by the FDA.
Because Elidel acts as an immunosuppressant, the medication is advised to only be used as a last resort for patients suffering from atopic dermatitis. Elidel should be used in minimal doses and over short periods of time to decrease the risk of Elidel cancer.
In addition the serious risk of Elidel cancer, the medication has also shown to cause an elevated risk for viral infection, chicken pox, shingles, and other serious skin conditions. Other adverse side effects reported with Elidel use include:
- Headaches
- Soar nose and throat
- Fever
- Bronchial discomfort
- Flu symptoms
Factors that Elevate Elidel Risk
Elidel can pose significantly higher risks when combined with exposure to natural and artificial sunlight. The body’s compromised immune system, while on the medication Elidel, can be adversely affected when exposed to excessive light, making the patient more susceptible to the onset of Elidel skin cancer.
In addition to the serious risks linked to Elidel sunlight exposure, the FDA has also advised that the drug not be taken by:
- Children under two: In studies assessing the effects of Elidel on children and infants under two, there was an increased instance of upper respiratory infection.
- People with infected eczema
- Women who are pregnant or breastfeeding.
Discussing Elidel With Your Doctor
It is important to discuss the side effects, risks and benefits of Elidel and all medications with your doctor, in order to make informed decisions regarding your health. Make sure to disclose any medical or personal factors to your doctor that may put you at an increased risk for Elidel cancer or other serious risks associated with the drug.
Important issues to mention may include high levels of sun exposure or if you are currently receiving light laser therapy treatment.
Contact Us
If you or a loved on has been injured by the prescription drug Elidel, you may be entitled to compensation for your pain and suffering. Contact us today to speak to an The Oshman Firm attorney handling Elidel cases. We will evaluate your case, free of charge, and help you determine the best way to protect your interests and maximize your claims.
